GMP mRNA Manufacturing
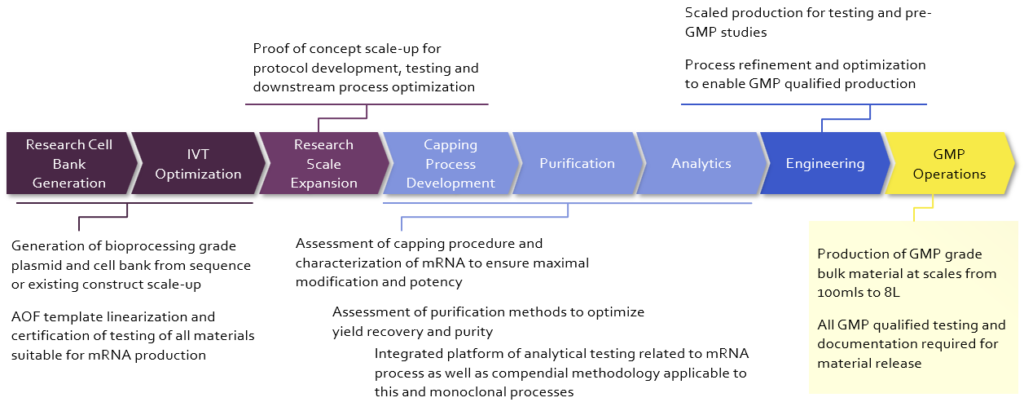
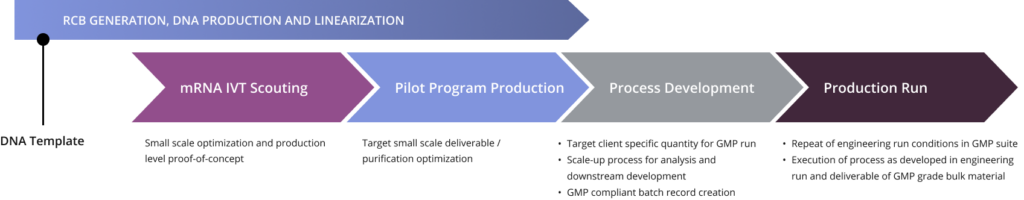
Curia’s experience in both chemistry and biologics has produced high quality mRNA up to 16 kb in length for the clinic. Our U.S. based end-to-end capabilities meet your manufacturing needs for batch release of mRNA drug substance and LNP formulation and fill/finish.
- GMP compliant, single use equipment
- Cell-free mRNA manufacturing
- Large scale production up to 40 L/batch
- ISO7 suite
- Expertise in self-amplifying mRNA production
- Onsite analytics for in-process testing and batch release