GMP Mammalian Manufacturing
Curia has facilities that can provide GMP manufacturing services. While they are generally served as part of our Integrated Solution packages, we can provide them as individual services.
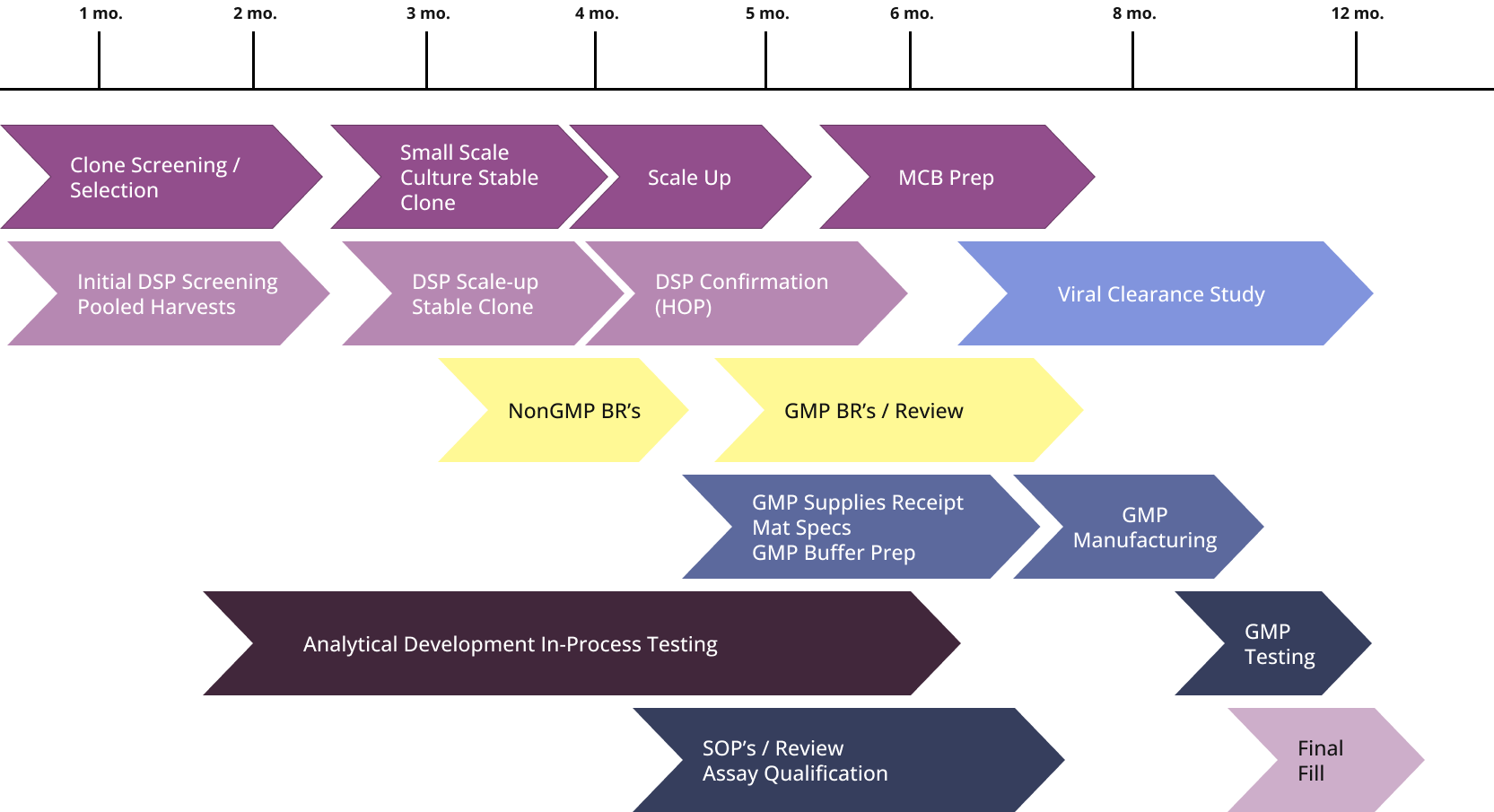
The Curia Hopkinton, MA facility is ISO 13485:2016 certified and provides GMP manufacturing of mRNA, antibodies, proteins, and vaccines and collaborates with Curia Hayward, CA for process development.

Antibody & Protein Therapeutic Development and cGMP Manufacturing
- Single use equipment
- 25L-1500 liter working
- ISO7 post-viral and fill/finish suite
- Onsite analytics for in-process testing and batch release
- Total timeline 9-12 months for phase 1 readiness