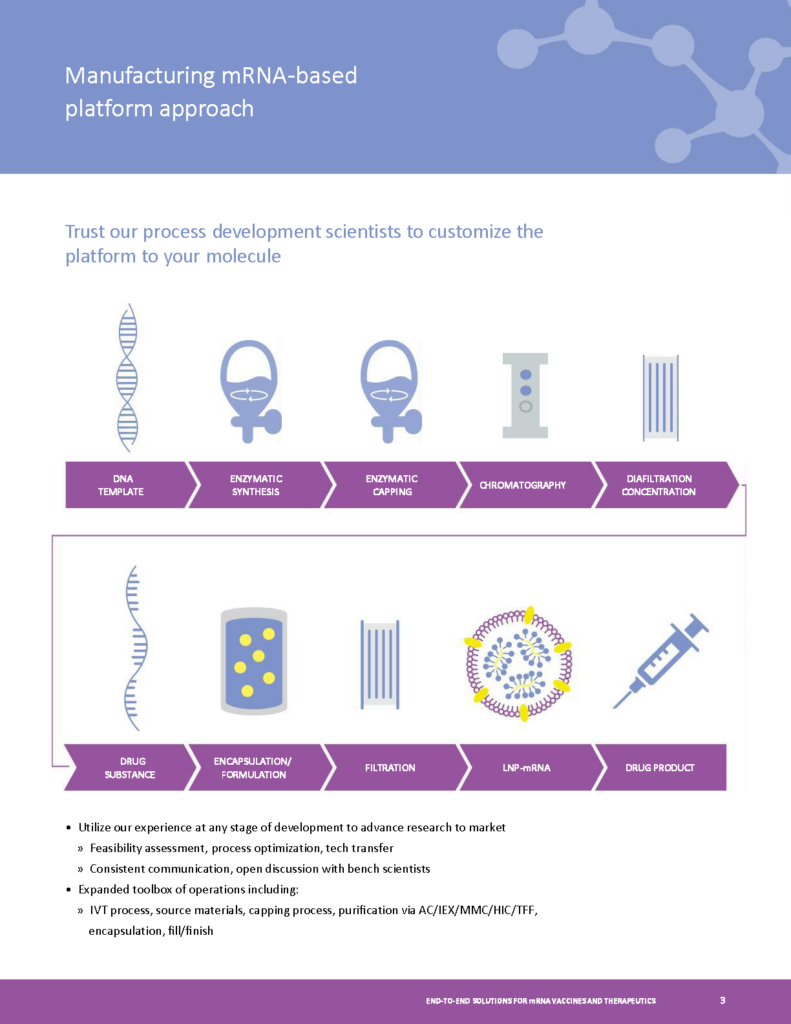
mRNA Process Development
Curia’s expertise spans discovery and engineering, mRNA drug substance, formulation and fill-finish, and manufacturing of lipids.

Highlights
- End to End solutions from DNA to Drug Product.
- Domestic Facilities with R&D, Analytics, GMP Manufacturing, Formulation, and Fill/Finish.
- Process Development to GMP Production of mRNA therapies.
- Small scale optimization, early stage material and GMP grade up to 40L per IVT batch.
- Curia has successfully produced high-quality mRNAs up to 16 kb for the clinic.
- Expertise in self amplifying technology and difficult to manufacture molecules.
- Full analytical capabilities for in-process testing and batch release of mRNA drug substance and drug product, including custom development to meet the unique qualities of your molecule as well as evolving regulatory requirements.