Cell Line Development
Curia offers advanced methods to rapidly move programs from DNA to RCB utilizing methods to ensure clonality and maximize titer. The TunaCHO® platform for transient production and the CHO-GSN® platform for stable cell line expression use the same CHO-K1 parental so products from the different platforms have similar activity & post-translational modification (PTM) profiles.
Developed from the same CHO-K1 parental cell line as our TunaCHO® transient production system, Curia offers the CHO-GSN® stable cell line expression platform for the production of the proteins and antibodies.
The CHO-GSN® platform provides a glutamine synthetase (GS) knockout cell line for the generation of a research cell bank (RCB) and future use as a manufacturing cell line for commercial products.
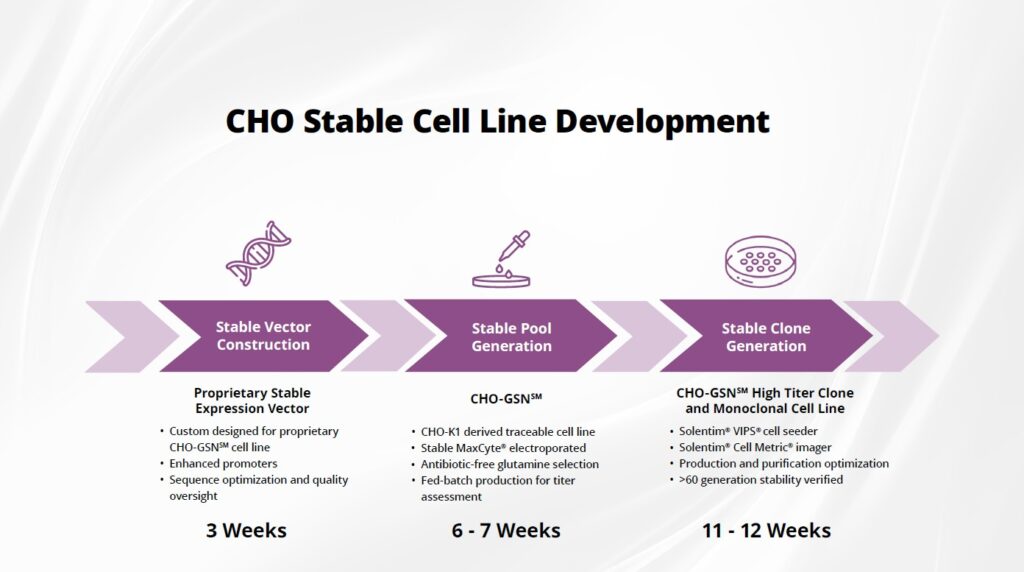
Curia’s CHO-GSN® CHO-K1 GS knockout for stable cell line generation is:
- Well characterized, providing the ability to enable a 12-month construct to clinic platform
- Compatible with chemically defined commercial media
- Robustly tested for bioreactor production and shear force tolerance
- Used in 100+ pre-clinical programs and clinical trials across various protein modalities (mAb, bi-specific, fc-fusion, enzyme)
- Cost effective with competitive with flexible licensing options compared to other premium CHO production systems
Curia’s Technological Approach

- Electroporation to ensure efficient delivery of desired genes of interest to the cellular fabric
- Single cell plating to isolate multiple high expressors
- Cellular imaging to monitor proliferation of a consistent monoclonal cell line
Features
- Stable transfections to CHO-GSN® and CHOZN® cell lines
- Proven stability >60 generations
- Single cell cloning and monitoring for production of stable research cell lines
- Documented progression of research to master cell bank implementation
“What sets Curia apart is that we have a wholly owned CHO-K1 GS knockout cell line (CHO-GSN®) that we control the licensing terms for. Our stable platform was derived from the same parental cell line as our transient platform (TunaCHO®). This enables our clients to begin their investigative studies transiently with the confidence that the findings will translate seamlessly into our stable platform, especially for post translation modifications (PTMs). Further, we offer cell line development, process development and cGMP manufacturing under one roof in Hopkinton, Massachusetts. This allows our teams to collaborate from the earliest phases throughout the entire program, creating efficiencies and eliminating tech transfers and delays.”
— Bill Hermans, GM and Site Head, Curia Hopkinton

