High Potency
Partner with us — we provide the most advanced technology for the production and safe containment of your highly potent compound. Our teams are experts in the cGMP production of highly potent compounds, from grams to hundreds of kilograms per year. We offer 30 years of expertise in the production, handling and containment of cytotoxic and other highly potent compounds.
Curia’s SafeBridge®-certified facilities include jacketed glass reactors, portable Hastelloy or glass-lined kettles, portable and fixed pressure filters/dryers, HPAPI isolators, as well as particle size reduction technology.
Capabilities
- State-of-the-art, SafeBridge-certified facility
- Equipment including cGMP-compliant glove box isolators and reactors capable of handling materials with OELs under 1 ug/m3
- Manufacturing capabilities up to 1200 L reaction scale with batch sizes of 50–100 kilograms
- Large-scale cytotoxic capabilities
Key Technologies
- Hydrogenation
- Fermentation
- Biotransformation
- Flow chemistry
- Micronization
- Lyophilization
- Emulsions
- Suspensions
Capacity and Capability
Curia supports each stage of the highly potent product lifecycle. Whether you are embarking on your inaugural high potency program and need a consultative guide through the process or you simply need a strong partner to support your latest project. Our team has the flexibility, experience and expertise tailored to your need.
High Potency Experience
- Steroids (unique high potency capability)
- Hormones
- Complex small molecule development
- Antibody Drug Conjugates
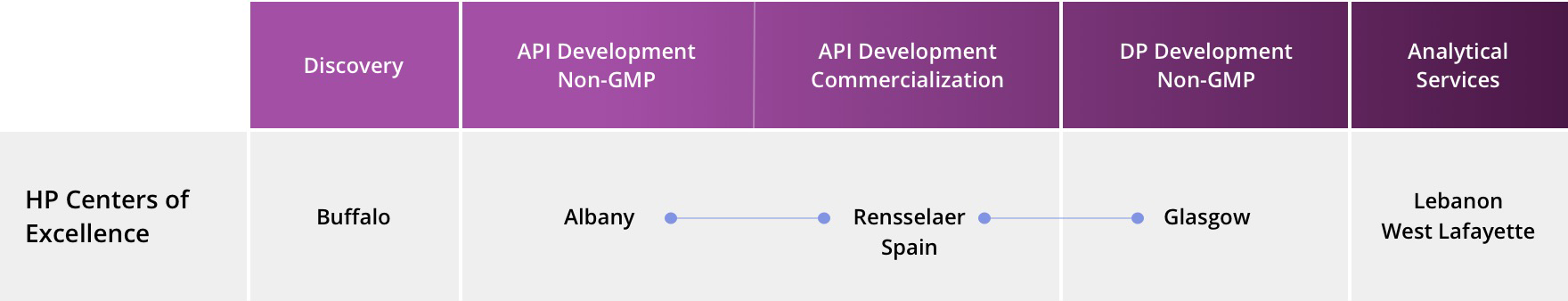
High Potency Expertise from Discover to Drug Product Development, Bolstered by Analytical Capabilities