Chemical Process R&D
Our chemical development team, comprised of more than 150 scientists in four countries, exceeds expectations on even the most challenging projects. Working in state-of-the-art laboratories equipped with the latest process and analytical instrumentation, we efficiently conduct route scouting, rapid process development, optimization of reaction conditions for scale-up of materials for preclinical trials or large-scale manufacturing non-GMP and GMP. With support from our expert team of analysts, chemical engineers and QA professionals, we rapidly and efficiently develop scalable manufacturing processes to meet any need, including development of high-potency APIs and DEA-licensed controlled substances.
Coupled with the synthesis of process intermediates and GMP raw materials, we also offer cGMP manufacturing of your API in small scale and in production scale. Curia handles small-scale API production critical for discovery and development, GMP scale in Albany and manufacturing at our global API facilities. We handle your cGMP manufacturing needs seamlessly from grams to metric tons — and through all phases of clinical trials — without the need for technology transfer to another company. Whether you require the preparation of API for toxicological studies, Phase I–III clinical trials or commercial supplies, we leverage the expertise and technology to make your project a success.
Curia has the infrastructure to perform rapid scale-up. Our multiple kilo-scale laboratories are equipped for the preparation of cGMP starting materials and other process intermediates on a scale from hundreds of grams to tens of kilograms.
Our capabilities include:
- Multiple jacketed glass reactors from 10 L up to 3000 L capacity
- Large-scale hydrogenation capabilities (up to 80 L cGMP and 1000 L non-cGMP)
- Synthesis at extremely low or high temperatures with up to 1000 L (-100 °C to 200 °C)
- High-pressure reactions (> 100 psi)
- Large-scale purification equipment (Biotage)
- Dedicated non-cGMP and cGMP high-potency areas
- Distillation capabilities up to 100 theoretical plates
- Development and optimization of biocatalytic processes from initial enzyme screening to implementation in large scale reactors
- Parallel screening equipment (EasyMax, HEL parallel reactor)
- PAT technology (e.g. ReactIR, FBRM, PVM)
- Jacketed reactors from 100 mL to 100 L
- Purification equipment (e.g. tangential flow filtration)
- Isolation equipment (e.g. filter dryers, spray dryer, centrifuge)
- Large scale chromatography – up to 70 L
- Access to full compliment of analytical equipment


Expertise in chromatography is critical for the purification of products from the fermentation broth, which require technologies that provide solvent usage. Our chromatography purification capabilities offer unique advantages, resulting in faster and robust processes with high purification of desired products.
We are skilled on normal phase chromatography and reverse phase chromatography. Solvents, mobile phase gradients, and flow rates are optimized to establish optimal peak separation.
The scale up of the chromatography is optimized considering parameters such as mobile-phase polarity, column loading, column height versus diameter, flow rate, pressure and temperature. In addition to careful optimization, large-scale chromatographic separations require specialized technology. Our equipment includes solvent reservoirs connected to fluid pumps through in-line flowmeters, static mixers, switching valves, and columns.
- Typically used if we cannot purify compounds by re-slurry/crystallization techniques
- Experience in developing TLC conditions to separate compound/impurities on normal/reverse phase systems
- Test conditions on Combiflash automated purification systems
- Experience in purifications using ion exchange chromatography
- For concentration of fractions we typically can use multiple 20L rotovaps in parallel and up to a 100L reactor (40L/h)
- Can also use TFF unit to concentrate fractions that have an aqueous component and a tray lyo that can lyophilize ~22.5L vol
- Analytical team available to support fraction purity analysis and ability to use flexible collection criteria to support early phase clinical batches
- Biotage Flash 400 (non-GMP)
- Can support 20 kg & 40 Kg cartridge
- Can isolate up to 8 kg of product at 6L/min
- Also have Biotage 150L & 75 L/M systems
- Biotage Flash 400 (non-GMP)
- Can support 20 kg & 40 Kg cartridge
- Can isolate up to 8 kg of product at 6L/min
- Also have Biotage 150L & 75 L/M systems


For Reaction Calorimetry:
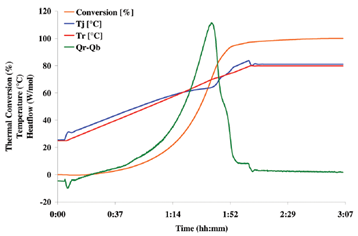
- Two RC1e reactors (-40 to 150 °C, Vmin 200 mL, Vmax 800 mL, Glass and Hastelloy C22 6 bar) -> heat of reaction, adiabatic temperature rise, reagent accumulation and gas generation measurement.
For Thermal Stability:
- DSC (Differential Scanning Calorimeter) -> decomposition onsets and energies
- TSU (Thermal Screening Unit) ) -> decomposition onsets and pressure rises
- ARC (Accelerated Rate Calorimeter) -> Time to maximum rate (TMR)
- VSP2 (Vent Sizing Package 2) -> Runaway reaction investigation & emergency relief sizing.
For Explosivity Screening:
- DSC [pre-screening tool for potentially explosive compounds (Yoshida’s correlation)]
- BAM Fall hammer for impact sensitivity measurements
- BAM Friction apparatus for friction sensitivity measurements
For Dust Explosion Hazards:
- MIE (Minimum Ignition Energy) -> Determines how easy a dust can be ignited.
- Chargeability and Charge relaxation -> Can a dust become statically charged and
Expertise in continuous flow chemistry is critical for the development and manufacture of intermediates and APIs, which require technologies that provide safe energy and solvent usage. Our continuous flow processing capabilities offer unique advantages compared with batch processes, resulting in faster, safer and more robust material production with higher selectivity of desired products.
Curia’s facilities are built to suit the chemistry. Our expert equipment and capabilities include:
- Tubular reactors and static mixers to handle multiple batch sizes (mg to kilogram scale)
- Reactions that require micromixing
- Commercial units including a Corning reactor, H-Cube for hydrogenations and custom-built reactors for high-temperature reactions (100–300 °C)
- Ability to scale extreme temperatures (reactions from -78 °C to 150 °C using tubes and tube-in-tube reactors)
- Ability to scale hazardous chemistry
- Access to microwave, photochemistry, electrochemistry and sonochemistry
- GMP Manufacturing (Phase 1-3)
- Preparation of APIs for Nonclinical Safety Studies
- Optimization of Reaction Conditions
- Critical Process Parameter Assessment
- Controlled substances capabilities
- De-novo route design
- Late Phase Process Development
- Process Understanding/Intensification
- Focus on robustness, quality and control
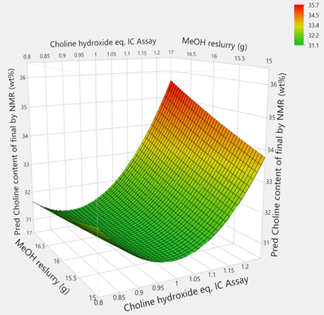
- Design Space
- Understanding the limits (edge of failure)
- Use of DoE, process modelling
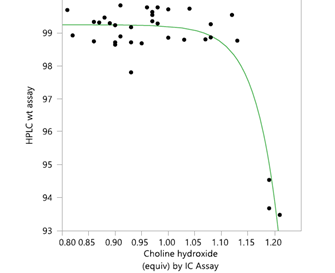
- Impurity purging
- Greatly helps in setting specifications in raw materials/intermediates
- Range Finding Studies
- Proven Acceptable Range (PAR) and Normal Operating Range (NOR)
- Critical Process Parameter Evaluation
- Registration/Validation (Technology Transfer)
- Risk Assessment
- Post commercial process development
- Process Understanding/Intensification