mRNA Process Development
With our scale and experience in both chemistry and biologics, Curia is uniquely positioned to provide mRNA drug development solutions.
Our expertise spans discovery and engineering, mRNA drug substance, formulation and fill-finish, and manufacturing of lipids and nucleosides/nucleotides.
Curia has successfully produced high-quality mRNAs up to 16 kb for the clinic.
We have analytical development capabilities for in-process testing and batch release of mRNA drug substance and drug product, including custom development to meet the unique qualities of your molecule as well as evolving regulatory requirements.
Manufacturing mRNA platform-based approach
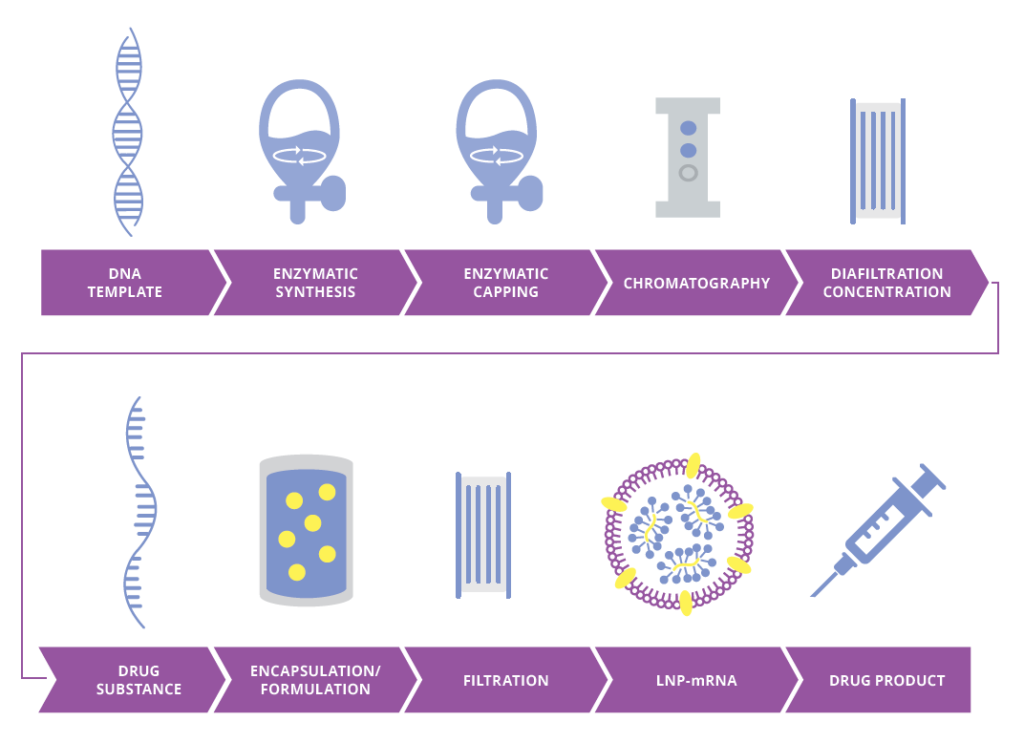
- Utilize our experience at any stage of development to advance research to market
- Feasibility assessment, process optimization, tech transfer
- Consistent communication, open discussion with bench scientists
- Expanded toolbox of operations including:
- IVT process, source materials, capping process, purification via AC/IEX/MMC/HIC/TFF, encapsulation, fill/finish