Developed from the CHO-K1 parental cell line, Curia offers the CHO-GSN℠ stable cell line expression platform for the production of proteins and antibodies. CHO-GSN℠ is a glutamine synthetase (GS) knockout cell line for the generation of a research cell bank (RCB) and future use as a manufacturing cell line for commercial products.
- Royalty-free, no commercial milestone payments
- CHO-K1 GS knockout with stronger GS selection, high titers
- Generational stability >80 generations demonstrated
- Established track record; multi-programs in clinical stages
As a service, RCBs are generated under the following workflow
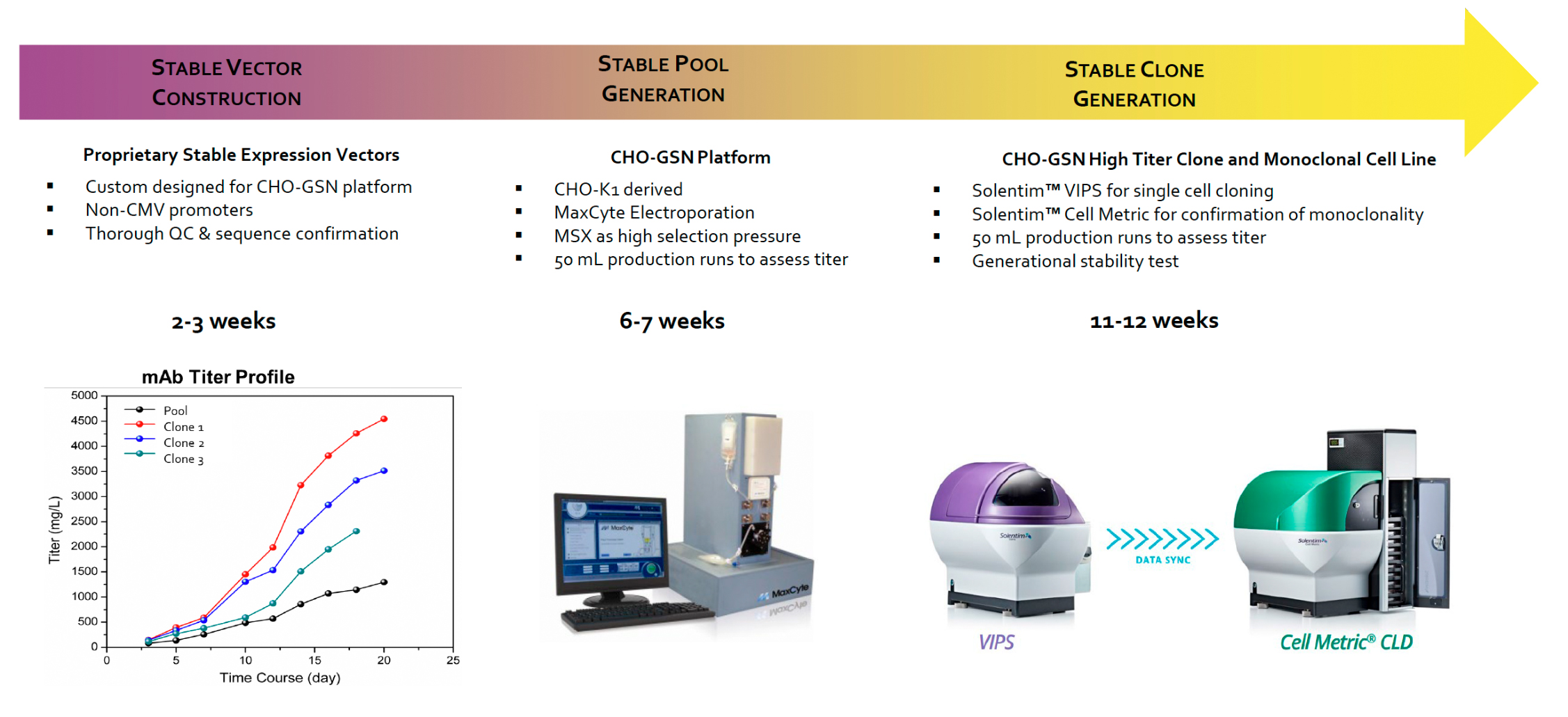
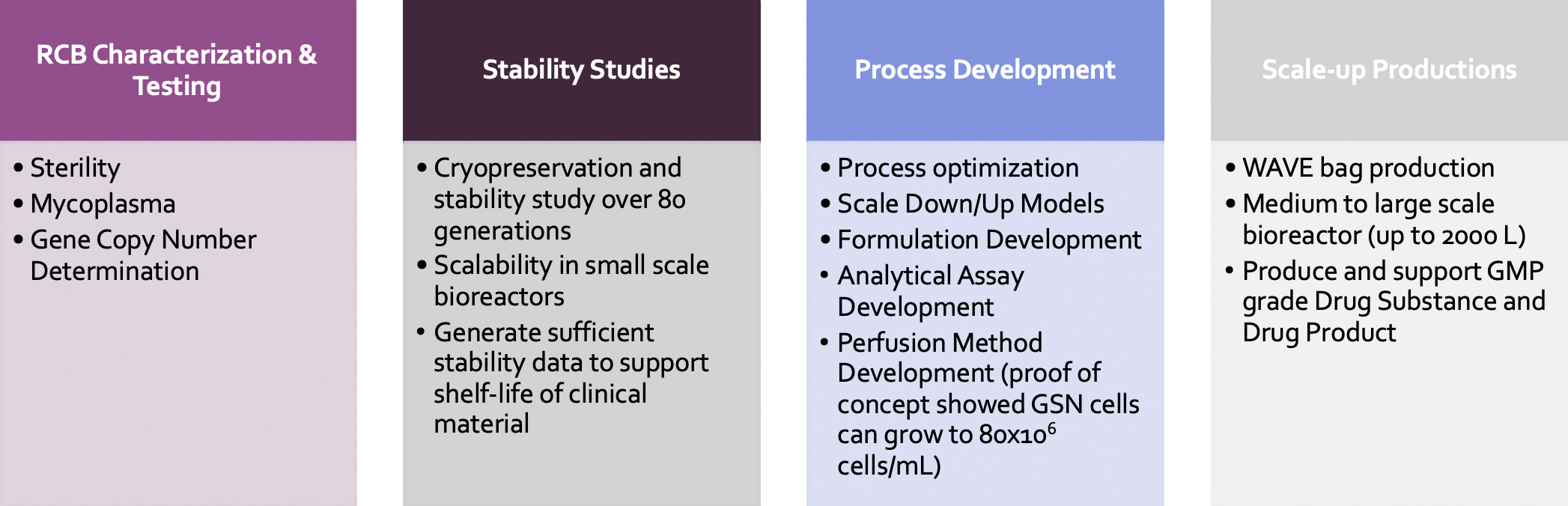
After the RCB is developed, Curia offers characterization services, MCB generation, and process development service
To learn about Curia GMP manufacturing for antibodies/proteins and mRNA, please click here.
Are you looking to accelerate your drug development program? If you would like to speak to a member of our expert team, please contact Curia here.
To learn more about these services, contact us to discuss your needs with an expert or fill out one of our Sample Submission forms.